#17 Flow: Making tDCS an accessible treatment for depression
The "D2C Bridge Strategy", Affordable Business Models and Making Something People Love
Hi friends,
I’m always yapping on about the need for more innovative treatments in mental health.
Well today, I’m very excited. Why? Because we’re going to explore a company that has not only delivered an innovative treatment for depression but has also built an impressive business around it.
In this post, we dive deep into Flow Neuroscience.
Over the last two months, I’ve got to spend some time with Erin Sivyer Lee, the CEO of Flow. I learned a lot about the Brain Stimulation market, what’s special about Flow, why they want to make tDCS an accessible treatment option for depression and their strategy for making that happen.
I hope you enjoy reading this post as much as I enjoyed writing it!
And if you do enjoy this post, feel free to share it with someone who would find it useful.
If you haven’t already heard of Flow, they are a small company from Sweden that has developed a Transcranial Direct Current Stimulation (tDCS) headset used to treat depression.
Over 30,000 people across Europe have used Flow and they’ve built a strong evidence base around their treatment - a 2023 RCT showed that 57% of patients using Flow went into remission from depression within ten weeks.
They created a successful Direct To Consumer (D2C) offering which acquired thousands of customers. Then, they leveraged that success to sell to private clinics and government customers. They’ve landed contracts with several NHS trusts and have demonstrated strong adoption with both clinicians and patients.
Impressive stuff.
Their story is full of lessons for mental health businesses and I’m pretty excited about sharing them in this post.
We’ll learn about;
- Becoming Part of the Depression Treatment Pathway. How Flow are working with the health system to become an integral part of the depression treatment pathways (and why that is so important).
- The “D2C Bridge" Strategy. Why Flow started off with a D2C Go-To-Market strategy and how they leveraged that to sell to big payers like the NHS.
- Making Something People Love. The piece most healthcare companies miss and how it benefits your strategy.
- Flow’s Sweet Spot. As a neuromodulation device company you usually get to choose between low prices, or a robust evidence base. Flow have both.
- A Business Model Focused on Affordability. Flow are focused on making their treatment as accessible as possible, and that means as affordable as possible. They actually deliver on that promise by building their entire business model around it.
- The Future of Flow. White space, the US and beyond.
Let’s get into it.

Understanding Flow (and why they’re important)
Two treatments dominate the current approach to depression; antidepressants and psychotherapy. While both of these can work for many people, they are far from perfect treatments.
- The efficacy of antidepressants is limited. “Antidepressant first-line treatment is effective in only around 37% of people” and “when compared with placebo, the incremental effectiveness of antidepressants may be modest, with only one in nine patients experiencing a benefit”. (1)
- Antidepressants can have a bunch of adverse side effects and are not suitable for everyone. (2)
- Psychotherapy has recovery rates of about 50% for those who complete treatment, but 60% of people drop out after two sessions or less. (3)
- Psychotherapy is also very expensive.
So my yapping for the introduction of more effective treatments is justified.
There is however, a third treatment option… Brain Stimulation.
Brain Stimulation has been around for decades in various forms, but it’s never reached mass adoption. Flow have set out to change that with their tDCS headset.

Patients use the Flow headset in 30 minute sessions over multiple weeks to reduce their symptoms of depression. The headset sends a weak current (1 or 2 mA) through the brain, gently stimulating the left dorsolateral prefrontal cortex (the area responsible for regulating emotion). In the brains of patients with depression, these areas are often less active than usual and tDCS works by restoring brain activity in them.
Now, the question I had when I started looking at brain stimulation devices like Flow was, does this actually work?
tDCS as a treatment has a strong evidence base. Like most treatments however, there are some studies that challenge it’s efficacy. But these studies are often for different treatment protocols to what Flow offers. To know if Flow works, we need to look at the specific trials completed using Flow’s product and protocol. When we do, we see some very impressive results.
An RCT from 2023 comparing Flow to a placebo found that 57% of patients went into remission within ten weeks and there were no serious side effects. The paper concluded that;
“Home-based tDCS with remote supervision is a potential first line treatment for MDD [Major Depressive Disorder] that is acceptable and safe.“
When we look at the effect of Flow’s protocol during that study and compare it to a meta analysis of the twenty one best-selling antidepressants, we can see that it is over twice as effective (and without the side effects of those drugs).

So there’s really strong evidence that Flow’s headset and protocol can be an effective treatment for depression. It’s this evidence that has given the NHS confidence to offer Flow as an option for many of their patients.
But Erin recognises that having an effective treatment is really just the first step in achieving the impact she envisions for Flow. In our conversations, Erin constantly spoke about accessibility. And one critical component of making Flow accessible, is getting it adopted into the treatment pathway for depression.
1. Becoming Part of the Depression Treatment Pathway
Flow’s north star is to get tDCS adopted into the treatment pathway for depression around the world.
So what does it actually mean to make that happen?
It means every clinician needs to see Flow and tDCS as a legitimate option for the treatment of depression. It means every patient needs to be open to it and able to use it effectively. It also means showing an impact, safety profile, cost saving, and affordable price to payers so that they will reimburse it.
While antidepressants and psychotherapy are already at the core of the treatment pathway for depression, tDCS still has a long way to go.
But Erin and the Flow team have a clear plan on how to get there and it revolves around three pillars;
- Building trust. Flow needs clinicians to have a deep trust in their product in order for them to offer it to patients. Likewise, Flow need patients to trust that their device is safe and will work. They have conducted a bunch of research to prove the efficacy of their product and build this trust - and they will continue to do so. But as I’ve mentioned before, while research is at the core of building trust, it is not enough. Not everyone is going to read your research papers, so building a credible brand, with lots of social proof is also needed. Flow have been really impressive in this regard, using lots of customer and provider testimonials, review scores and emphasising the partnerships they have with trusted organisations like the NHS. As they build trust over time, more providers will offer Flow as a treatment option to patients and more patients will accept it (or demand it themselves). This gives Flow more funding which can be used to conduct more research and build a stronger brand, accelerating the flywheel of trust.
- Driving price down. In healthcare, “accessible” is essentially a synonym for “affordable”. Unless Flow’s product is affordable and saves payers money, they aren’t going to pay for it and it won’t become part of the treatment pathway for depression. Flow have built their entire business model about making their treatment affordable - see below.
- Increasing usability. Although anti-depressants have some drawbacks, they are extremely easy to use. Flow must get close that level of usability in order to become part of the treatment pathway. But delivering treatment through a home-use hardware devices comes with a set of very “real world” problems. It’s great to have a headset that works in a research environment, but how do you make sure people can effectively use it themselves, at home? How do you make sure they get their headsets easily and on time? How do you make sure they don’t mess it up when they use it? Flow have implemented a design focused methodology to tackle this problem that I see in very few other mental health businesses. They’ve made consistent design decisions to prioritise usability - sometimes having to say no to attractive options like increased personalisation. They’ve built logistics and customer support systems to make sure people can easily get their headsets and that they work for them. Sounds simple, but it’s this non-sexy stuff that will be critical to increasing the adoption of Flow within Health systems. Nobody will adopt a treatment that is a faff to use.
Yes, tDCS can work as a monotherapy, but Flow aren’t trying to replace antidepressants or psychotherapy. In fact, Flow has strong evidence working as an adjunctive treatment.
Studies have shown that combining tDCS with both antidepressants or psychotherapy can improve outcomes for patients more than either of those therapies in isolation - an important proof point in driving adoption. It’s all about giving clinicians and patients more options.
Flow have a treatment that works and want it adopted by health systems as part of their treatment pathway for depression. To achieve that long term goal, and to be a successful business, they need to land big payer contracts.
They’re already well on their way to making that happen, and it’s a counterintuitive Go-To-Market strategy that has allowed them to do it…
2. The “D2C Bridge Strategy”
Shall we talk about one of my favourite topics for a moment? Yep, I want to talk about Go-To-Market strategy.
This is one of the biggest set of questions for a Mental Health startup… Which customers should I go after? How do I acquire them? Should I go D2C? B2B? B2B2C? B2G2B2C?
When your Go-To-Market strategy starts sounding like a postcode, you know you have a problem…
Flow implemented a counter-intuitive Go-To-Market strategy that I am seeing more and more successful healthcare companies adopt. It’s the strategy we used when I ran Growth at LetsGetChecked. It helped us scale and become a unicorn and it’s now doing the same thing for businesses in mental health.
I call it the “D2C Bridge Strategy”.
It’s based on a very simple realisation most healthcare founders have;
“Big payers (like Governments) are the ones with all the money and incentives to pay for my product. But they’ll never buy from me until I’m much bigger and have stacks of evidence”.
and…
“The only people I could sell to today are a small segment of consumers, but that will never be enough to have a big business.”
In metaphorical terms, there’s a land of scale and profitability inhabited by BPCs (Big Payer Contracts). Between that land and the land where startups begin their journey, there’s a wide river filled with demands for evidence, credibility and “bigness”.

So how do you get over the river?
You build a D2C Bridge.
Here’s what the playbook looks like;
- Make a consumer product suitable for D2C
- Make your brand attractive to consumers (check out Flow, Mindset Health, Flo (the other Flo) or LetsGetChecked to see what that looks like)
- Build a D2C Go-To-Market motion (paid ads, landing pages, reviews etc.)
- Use your initial customers to start to build your evidence base (even it’s initially just from customer surveys) and ideally run actual trials
- Use this traction and any early evidence to build your brand further (more reviews, customer testimonials, research papers etc.)
- Use your newfound brand awareness and evidence base to open up conversations with payers
- Raise funds from investors on the back of your your D2C traction (hopefully a nice chart up and to the right) and these early opportunities you’ve created with payers
- Close initial payer contracts but keep D2C rolling
- Over time, grandfather the D2C channel as it’s eclipsed by BPC revenue.
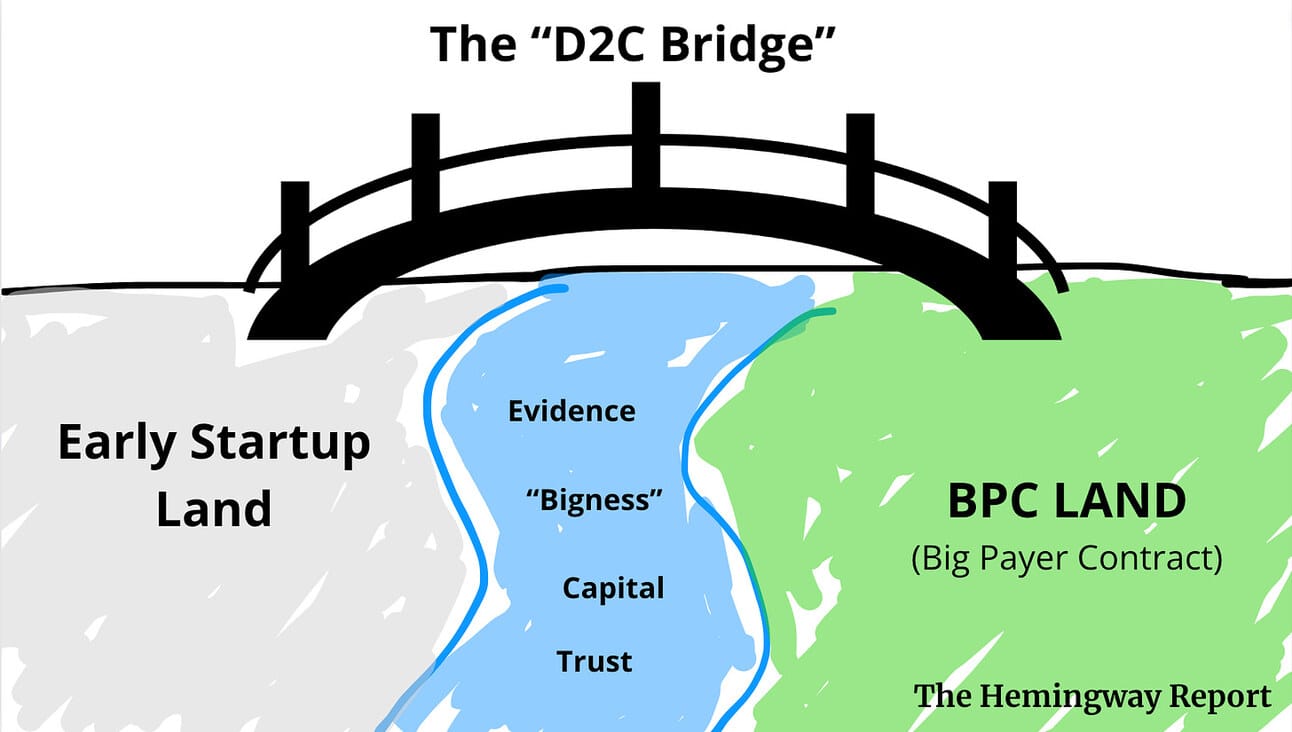
Flow executed this playbook perfectly.
Like any startup, when Flow was first founded, they had no customers, no evidence base and no chance of convincing someone like the NHS to adopt them.
So what did they do? They built a D2C offering.
For years, 100% of their customers and revenue came from D2C. They built their evidence base and brand awareness to the point where they could open up conversations with big payers like the NHS. Clinicians and NHS employees had already heard about Flow because of their traction with patients and the attention they had got in the media. Ultimately, that led to Flow closing deals with several NHS trusts.
Today, instead of 100% of their revenue coming from their D2C channel, it’s only 30%, the remaining 70% coming from clinics and reimbursed channels like the NHS.
So that’s the playbook. Run it. Build a D2C bridge and walk over it into the land of BPCs.
3. Making something people love
There’s something I’m often guilty of… focusing too much on the systems of healthcare and not enough on the individuals in that system. Specifically, the patients.
We can become so focused on trying to navigate payer landscapes, Go-To-Market plans, capital raising and running research that we have little time left to think deeply about the people actually using our product. What are they going through? What does their experience look like? How do we make our product better for them, even in a tiny way?
This leads to a criticism I have of mental health startups; they don’t build something people really love. Something that is easy to use, that meets the high expectations people have for product experiences and that makes their life genuinely better.
Flow are an exception to this criticism. And while the Y Combinator advice of “build something people want” seems obvious. We can learn a lot from a company in mental health who has actually managed to do it.
When I was researching Flow I spent a lot of time reading reviews, forums, social comments and Reddit threads. Here’s a review for Flow posted just twelve hours ago;

People genuinely seem to love Flow.
They’ve got almost 400 Trustpilot reviews and a rating of 4.4 stars. That’s not normal for a healthcare treatment company.

Of course, if you are a healthcare company without an evidence base, this “love” is pretty worthless. But there are also a bunch of companies who built a strong evidence base and failed. Why? Because even though their product worked in research environments, without being loved, they could never get it adopted by users at scale.
Flow get this.
They have made their user experience a real priority at the business. They understand their user, they prioritise them and focus on improving their experience in whatever way they can. Just look at this review.

Notice how easily and quickly they replaced the headset. And for free. Does your business meet that level of service for your users?
Flow know how to create great experiences. And they reap the commercial rewards of doing so. Within the NHS, their data showed that over 90% of NHS patients chose Flow vs 10% who chose antidepressants. Like we saw in my analysis of Pear and Akili, it’s not enough just to have your treatment reimbursed, you need to get clinicians to recommend it and most importantly, patients to actually use it.
Erin thinks their Swedish roots have a lot to do with their ability to create great customer experiences. Sweden is known for it’s cultural focus on design and is the home to top-tier consumer companies like IKEA, Volvo, Spotify and Minecraft. This culture permeates Flow and drives all of their decisions. Competitor products have complex instructions, even requiring users to mix their own saline solution and place the electrodes in the right place on their head themselves.
That’s not the case for Flow. The headset itself has been designed from start to finish to be incredibly easy to use; there’s not a single thing on the headset that doesn’t serve a purpose. And users love that.
Whether it’s the Swedish influence or something else, they’ve built something users love and (cheesy as it may sound) are changing people’s lives.
I mean, isn’t that what we’re all trying to do here?
4. Flow’s Sweet Spot
The Brain Stimulation market is not new. There are a few different treatments for various mental disorders'; from Electroconvulsive therapy (ECT), Repetitive Transcranial Magnetic Stimulation (rTMS), Vagus Nerve Stimulation (VNS) and more. I won’t attempt to provide an overview of the entire Brain Stimulation industry in this post (if you want one focused on TMS, check out this post by Naveen Rao).
Many smart people have seen the potential for Brain Stimulation therapies and have built companies to pursue that opportunity.
But Flow have found a sweet-spot for themselves in the market, one that makes them a compelling option for patients, providers and payers.
We can classify the Brain Stimulation market for depression across two dimensions; (1) price and (2) level of evidence / regulatory rigour.
There are lots of low cost tDCS headsets that you can buy, starting from around $100. But they have essentially no evidence, zero regulatory rigour and as a result, will never be adopted by clinicians.
Some other Brain Stimulation devices have built strong evidence around their product and have gone through the necessary regulatory hurdles. But they are incredibly expensive, often comparable to rTMS treatments with prices upwards of $5,000.

Flow’s secret has been to find the sweet spot between both of these camps, building a robust evidence base and taking the product through the regulatory process whilst maintaining low prices.
The truly special thing about this sweet spot is that it allows them to aggressive and creative with pricing.
Because their COGS are small, they can offer low initial prices to payers which drives early adoption. Then, because they have faith in their ability to deliver outcomes for patients, they can implement a performance-based or revenue share model. This is an extremely attractive offer to payers which helps Flow land contracts, but because they can actually deliver on patient outcomes, they can still make great revenue.
Looking at this sweet spot, we’ve already discussed how Flow built their evidence base by investing in research and building a “D2C Bridge”.
But how have they managed to maintain low prices?
5. A Business Model Focused on Affordability
The first cause of death highlighted in Why Mental Health Companies Die, was Business Model Failure. More specifically, mental health companies die because they don’t do these three things;
- Know who their customer is and what they value
- Know how to deliver this value to them
- Deliver this value at an appropriate cost
When you know these three things and build your business model around them, it creates powerful alignment across your entire organisation.
It becomes the hymn sheet from which everyone sings, producing a harmonious and powerful tune.
If Flow had a hymn to sing, it would be called “Affordable Grace”.
Get it?
No? Ok… Moving on….
I’ve already Erin’s desire to make Flow accessible to as many people as possible and how affordability is at the core of making that happen. But doesn’t every mental health company want this? For their products to be accessible to the masses? To be able to offer it at low prices?
The difference between Flow and other companies is that they’ve translated their desire for accessibility and affordability into action, and they’ve done this through business model design.
Let’s look at how Flow stacks up against the three components of a successful business model;
- They know who their customer is and what they value;
- Patients with depression want to get rid of their symptoms
- Payers responsible for those patients want the same thing, but need to do so in a way that fits within their system and healthcare practitioners
- They know how to deliver this value; they have an effective, trusted, easy to use treatment
- But most importantly, they have deigned a business model to deliver this value at an appropriate cost.
Flow have taken their ambition to be affordable, to “deliver their value at an appropriate cost”, extremely seriously. They designed their business model and culture around it and are executing on this strategy by making decisions every day, across all departments.
Here’s some examples of those decisions;
- They kept their team super lean, they still only have twenty people
- They stripped back their product to just the core features, saying no to additional options like allowing for increased personalisation
- They don’t have a big sales and marketing team
- They’re pursuing scale economies to drive down manufacturing costs
- They’re ruthless on capital allocation
- They invest today in infrastructure that will drive costs down tomorrow. Before they needed it, Flow built a support system that allows them to employ just one support staff for over 30,000 patients and over 300 clinics.
In Erin’s words;
“People would be surprised how small our team is. That's a product of ruthless prioritisation and focus and just really incredible individuals on the team.
We've also been really thoughtful about how to design our product, which means there is nothing on that device that doesn't serve a purpose.
And in general, we are just extremely thoughtful about where we invest our dollars.”
This business model also creates a flywheel that brings costs down further over time;
“The cost of manufacturing is quite low, which means we can be very aggressive on pricing so that people can experience the delight of Flow, which helps us with growth, which helps us with volume, which helps us with cost. So we really do see it as a flywheel in that way.”
Everyone wants to be accessible.
Most want to be affordable.
Few design their business model around it.
And almost nobody executes on that strategy by making tough decisions every day.
You can buy a Flow Headset today for just €469 ($520) - about the price of three therapy sessions.
This low price, coupled with the efficacy of their treatment has allowed them to create impressive cost savings for health systems;
- £4,400 Annual savings per patient vs. existing UK standard of care (i.e. combination of antidepressants and talk therapy)
- £10,000 Annual savings per patient in Community Mental Health
- £1,032 First-year savings per patient vs. Antidepressants (primarily from a 40% reduction in visits otherwise needed for managing drug titration, medication reviews, and side effects)
Over time, Flow’s ambition is to continue to drive this price down.
It will enable more payers to reimburse it, providers to offer it, and patients to use it, ultimately achieving their goal of accessibility and having Flow adopted as part of the treatment pathway for depression.
The future of Flow
The white-space in front of Flow is ginormous.
While tDCS continues to be explored for clinical applications like schizophrenia, addiction, epilepsy and chronic pain, Flow remain focused on depression. Unfortunately, there are still a lot of people struggling with depression who need access to better treatments. In the UK alone, 16% of adults experienced moderate to severe depressive symptoms in 2022. 8.6 million people were subscribed anti-depressants in across 2022 and 2023.
Flow offers a treatment that is not both effective, but affordable. It’s easy to use and without many of the side effects of other treatments. And it’s scalable.
Of course, cracking the US will be a major priority for Flow. While tDCS is not yet approved by the FDA as a treatment for depression, Flow hope to change that. The US will pose a very different challenge to Europe offers a huge prize if they can conquer it.
Their success will hinge on their ability to continue increasing efficacy, increasing their evidence base, driving costs down and executing a successful go to market strategy in new and existing markets.
Hopefully, they can follow in the footsteps of their fellow Swedish multinationals, building fantastic products, establishing global brands, and above anything else, making the lives of their customers better.
Thanks to Vooha Vellanki for her help in editing this piece.
If you liked this, feel free to share it with someone.
That’s all for this week.
Keep fighting the good fight!
Steve
Founder of The Hemingway Group
P.S. feel free to connect with me on LinkedIn
